Leveraging Cutting Edge Biotechnology Research in Japan For Stronger IP Portfolios and Research Prioritization
By Chie Mueller-Hillebrand Ph.D., David Adamovich Ph.D., and Drew Lowery, Ph.D.,
Global Prior Art
Japan is not the first country that comes to mind when Senior Managers and IP Attorneys within the Massachusetts Biotech sector discuss leading edge biotech research and its implications for research directions and IP. However, as the Nobel Prize awards to Tasuku Honjo (2018) and Shinya Yamanaka (2012) of Kyoto University clearly demonstrate, many breakthroughs in cancer therapies originated there. Much of these scientists early work, including research and patent filings, was published only in Japan, in the Japanese language, and not in English. As a result, U.S. companies that failed to monitor Japanese language patents and literature were blindsided by this work. In this article we highlight several fields where Japan is a leader in biotech research, and illustrate how accessing Japanese language documents facilities more effective decision making in such areas as R&D, licensing, and IP strategy.
Tasuku Honjo’s group discovered the function of Programmed Cell Death 1 (PD-1) in 1992, which subsequently led to the discovery of a whole new class of immune checkpoint molecules that regulate T cell activation. Some cancer cells are able to over-express PD-1 on their surface and evade the host immune response, leading to unchecked cell expansion. Since he was the first to discover PD-1, Honjo had a head start on everyone else for both developing and commercializing anti-PD-1 antibody therapy for treating cancer and filed early patent application to protect future market share. By 2005, a collaboration with Ono Pharmaceuticals and Medarex led to the development nivolumab which was approved for use in humans as a first in class cancer therapy (Opdivo) in 2014. By 2018, Opdivo became the top immune oncology drug in the world, with over $4.2 billion in sales in the US alone.
In 2006, Shinya Yamanaka made a discovery that revolutionized stem cell research and therapies. He found that a mixture of just 4 transcription factors — Oct3/4, Sox2, Klf4, and c-Myc — were sufficient to reprogram somatic cells into induced pluripotent stem cells (iPSCs). This discovery now meant that any research organization or therapeutics company would have the ability to generate their own stem cells for research rather than acquire cells sourced from embryonic or fetal tissues. With the knowledge of this discovery, a venture company originating at the University of Tokyo and Kyoto University founded ReproCELL, the first company to produce commercially available iPSCs.
To further illustrate these two breakthrough technologies which originated in Japan, we performed a high-level Japanese-only IP analysis of the cancer immunotherapy and stem cell technology spaces. This analysis relied on the HYPAT database, the most comprehensive source of Japanese language patents documents, and reviewed Japanese priority patents and applications that do not have English-language counterparts to identify filing trends that are missed when relying only on US and European patent information. All documents were searched and reviewed in their native Japanese language to avoid machine translation issues and ensure accuracy.
As expected given Honjo’s discovery of PD-1, there is significant filing activity in the immune checkpoint inhibitor space, and particularly in the area PD-1 antibody technologies (Fig. 1). Most of the early documents were focused on anti-PD-1 antibody monotherapy. as the therapeutic space has matured, however, applications are focused on combination therapies and developing the next generation of checkpoint inhibitors. Ono Pharmaceuticals is the most prominent player is this space (Fig. 2), and has a collaboration with Bristol-Myers-Squibb to produce Nivolumab. Other recently emerging cancer therapies, including cancer vaccines, adoptive T cell therapies, and oncolytic viruses, are also seen in the Japanese patent space (Fig. 2). Cancer vaccines generally involve administering cancer antigens to patients so that they can develop their own protective immune response to the cancer; prominent Japanese players in this space include OncoTherapy Science and BrightPath. Adoptive T cell therapies, which involve genetically modified T cells that express specific TCRs or chimeric antigen receptors (CARs), have sharply risen in popularity world-wide, including in Japan at Yamaguchi University, Takeda Pharmaceuticals, Noile-Immune Biotech, Mie University, Takara Bio, and Otsuku Pharmaceuticals. Work on oncolytic viruses (viruses that selectively infect and kill cancer cells) is being done primarily at Yamaguchi University and the University of Tokyo.
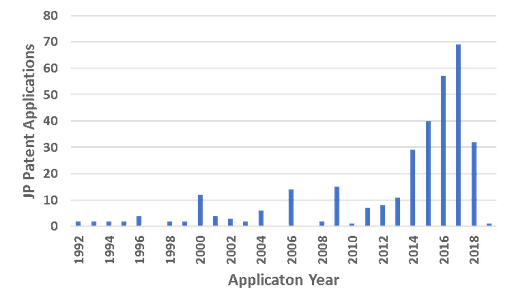
Fig 1. Numbers of PD-1 related patent applications filed in Japan (Please note that patent applications are published one and a half years after the filing date)
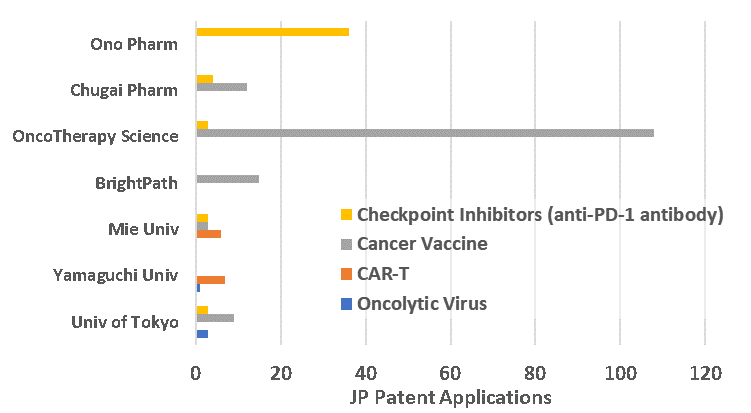
Fig 2. Numbers of Japanese patent applications of major focuses by players in cancer immunotherapy.
Thanks to the pioneering work of Yamanaka on iPSC technology, the Japanese government has supported R&D on iPSCs as a national project. In order to centralize and accelerate the development of iPSC technology, iPS Academia Japan Inc. (led by Yamanaka) was created to coordinate research capabilities, develop a resource covering world-wide information regarding iPSC research, and acquired a strong patent pool surrounding the technology. To facilitate commercialization, this company has acquired sub-licensing rights from multiple research institutes, including Kyoto University, which has a prolific portfolio in the space (Fig. 3). The patent portfolio covers everything from the preparation of cells (purification, genetic modification, reprogramming, and differentiation) to methods of culturing and expanding the cells, to therapeutic applications of the cells. Prominent participating Japanese assignees in the space include Sumitomo Dainippon Pharma, Takeda, Terumo, Riken, and Chiba University.
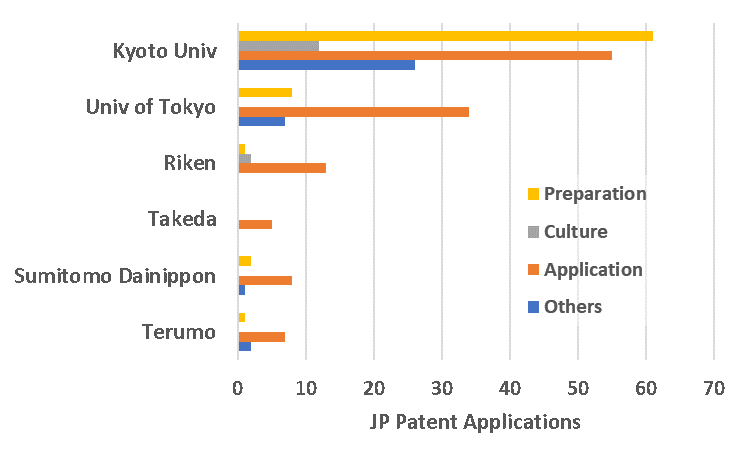
Fig 3. Numbers of patent applications of major focuses by players in iPSC technology
So why is access to Japanese language patents and technical literature important? The simple answer is that knowledge facilitates better decision making. Among Japanese patents and applications that lack a U.S. or other English language counterpart, about 22% relate to immune checkpoint inhibitors, 16% cover cancer peptide vaccines, 23% relate to iPSC technology, and 40% relate specifically to culturing iPSCs. If you are a VC or a Senior Manager in business development, that 22% of IP covering immune checkpoint inhibitors could yield the next billion-dollar drug like Opdivo. If you are handling IP Strategy related to inhouse work on iPSCs, that critical piece of technology and IP needed for commercializing your product may be available for license in Japan. If you are filing important patents, it is essential to identify and differentiate over highly relevant art published only in Japan. For a research group trying to make the next great discovery, the next lead may be found in a journal article from one of these Japanese Universities. Rather than operate blindly, accessing and leveraging cutting edge efforts in Japan allows prioritizing opportunities and accelerating R&D. The good news is that you do not require knowledge of Japanese to gain an edge, only a recognition of the importance of Japan’s biotech efforts in such fields as cancer immunotherapy or iPSC technology.








