Impact and Consequences of the Controversial Uncostitutional Patent term Extensions in Brazil: A Brief Analysis
By Giovanna Chinait, Director UNGRIA BRAZIL
The term available for patent protection in Brazil, and the potential extensions to such term, has always been a cause of debate in both the administrative and judicial instances.
In the most recent episode of this debate, the Brazilian Federal Supreme Court (Portuguese acronym, “STF”) ruled in favor of the Attorney General’s Office (Portuguese acronym, “PGR”), in connection with a case relating directly with the term extension of patents granted in Brazil.
In 2016, a Direct Plea of Unconstitutionality1 (Portuguese acronym, “ADI”) identified with No. 5529 was filed before the SFT, requesting the court to declare the unconstitutionality of the sole paragraph of article 40 of the Intellectual Property Law (Law nº 9.279/16), which was the only portion of said law that established a possible term extension of patent protection.
After five years of intense debate and several Amicus Curiae petitions in favor and against the ADI, in May 2021 the STF upheld the plea filed by the PGR and therefore considered unconstitutional, with ex nunc effects, the sole paragraph of article 40 of the Brazilian Intellectual Property Law.
The decision has been very controversial due to the existing patent prosecution backlog of the Brazilian authorities and the high profile of the involved parties. On one side, Brazil’s President, the Brazilian Congress, private pharmaceutical companies and the Chief Federal Attorney (Portuguese acronym, “AGU”) have defended the validity and maintenance of the challenged provision, while most of the associations of generic pharmaceutical companies kept challenging the article’s validity.
Considering the above-mentioned decision, and the fact that in Brazilian practice this provision was mostly used to strategically extend the protection term and exclusivity of patents, we will discuss some of the most relevant aspects surrounding the decision, as well as the historical context leading to the unconstitutionality of the article.
Sole paragraph of Article 40 of the Brazilian Intellectual Property Law Noº 9.279/16
The current Brazilian Intellectual Property Law, dated 1996, was issued to adapt the regime of Brazilian Intellectual Property to the Trade-Related Aspects of Intellectual Property Rights Agreement (TRIPS), which among others, established the minimum requirements regarding patent protection for its members, including a 20-year term protection from filing date, as well as rules regarding the protection of pharmaceutical products and processes.
As a result of the above regime change, article 40 of the amended Brazilian Intellectual Property Law included the protection term of invention patents and utility model patents as follows:
“Article 40. An invention patent shall remain in force for a period of 20 (twenty) years, and a utility model patent for a period of 15 (fifteen) years from the date of filing.”
Sole Paragraph. “The term shall not be less than 10 (ten) years for an invention patent and 7 (seven) years for a utility model patent, from the granting date, unless the BRPTO has been prevented from examining the merits of the application by a proven pending judicial dispute or for reasons of force majeure.”
The referred sole paragraph’s intention was to compensate, in favor of applicants, the excessive delay of the Brazilian authorities in prosecuting patent applications and aimed to guarantee a reasonable period of protection to avoid unjustified restrictions on the rights of holders. For instance, if the substantive examination of a patent application took more than 10 (ten) years, then the patent would be protected for an additional period of 10 (ten) years from the granting date.
Through this amendment, the provision would also be aligned to section 62.2 of the TRIPS Agreement, which states that country members shall permit the granting or registration of the applicable right within a reasonable period of time, so as to avoid unwarranted curtailment of the period of protection. Since Law nº 9.279/16 came into force, most patents were granted with a minimum term of 10 (ten) years of protection counted from the granting date, regardless of the prosecution term.
As of early 2021, it was estimated that between 45% and 47% of Brazilian patents would have been granted based on a minimum period of 10 (ten) years, which would result in an effective protection of more than 20 years. According to a research study conducted by the Federal University of Rio de Janeiro (Portuguese acronym, “UFRJ”) and published in 2020, the average period of time to grant a pharmaceutical related patent was 13 (thirteen) years, which in turn lead to an “effective” protection term of 23 (twenty-three years), considering the minimum protection period of 10 years set forth in article 40 of Law nº 9.279/16. Although the pharmaceutical related patent protection had an important backlog due to the Brazilian Patent and Trademark Office (“BRPTO”), this was also due to the fact that prior approval from ANVISA (Portuguese acronym for Brazilian National Health Surveillance Agency) was required.
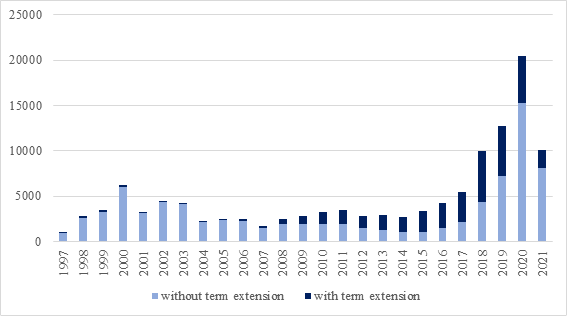
Figure 1: Patents Granted in Brazil from 1997 to the first quarter of 2021. Data obtained from the BRPTO website.
Brazilian Patent Backlog
The Brazilian patent prosecution backlog played an important role in the debates surrounding the amendments to the patent protection periods. Such backlog resulted in examination periods well in excess of 10 (ten) years, and as a compensation method, the sole paragraph of Article 40 of Law No 9.279/16 allowed patents to have protection terms of more than 20 years.
Since 2016, when the backlog reached its peak having 243,820 pending patent applications, the BRPTO had been implementing various measures to reduce the average time of patent application examination, such as the creation of several fast-track programs, joining the Patent Prosecution Highway (PPH) agreements and solving the problem related to the existing prior approval required from ANVISA . 2
These actions helped to reduce the backlog to about 149,930 patent applications pending of final decision by mid-2019. The most important effort to solve the past backlog came into effect in 2019, aiming to reduce the patent backlog by at least 80% by 2021 and enabling substantive examination to be carried along 2 (two) years, instead of 13 (thirteen) years 3.
According to BRPTO data, from January 2020, the average time for patent examination was reduced from 10 to 9.3 years which caused important changes in the strategy for patent prosecution in Brazil moving forward.
Currently, the backlog is comprised of 55,059 pending applications, out of which 47,073 applications are under examination and only 6,229 are ready for substantive examination, but have not issued an Official Action.
If the BRPTO is actually able to maintain the good results achieved by the before mentioned efforts, the sole paragraph of article 40 would have naturally fall into disuse.
Direct Plea of Unconstitutionality No. 5529
The Direct Plea of Unconstitutionality No. 5529 was filed by the PGR on May 17th, 2016 after various failed attempts before the Brazilian Congress to remove the sole paragraph of Article 40. In 2013, the Brazilian Association of Fine Chemistry, Biotechnology and its Specialties Industries (Portuguese acronym, “ABIFINA”) filed ADI 5061 for the assessment before the STF, requesting to declare the sole paragraph of Article 40 unconstitutional.
ADI 5061 argued that the provision of the sole paragraph would grant exclusivity for an undefined term causing uncertainty regarding the term of protection and this was contrary to Article 5, paragraph XXIX, of the Brazilian Constitution which states that inventions are entitled only to temporary protection. Furthermore, it argued that the possible undefined term would also cause a negative effect on social rights, including the right of access to public health. This first plea was dismissed by the STF, arguing ABIFINA had no legitimacy to file an ADI 4. However, ADI 5061 was used as a basis for ADI 5529.
Since the first procedural stages of ADI 5529, multiple authorities and industry associations, including patent holders (particularly holders of pharmaceutical and agriculture industry related patents), as well as civil society organizations that focus on advancing the right to health, filed various Amicus Curiae petitions, in favor and against the constitutionally of Article 40’s sole paragraph.
A preliminary injunction granted by the STF on April 7th, 2021, focused exclusively on pharmaceutical patents (products and processes) and medical equipment. The above injunction was granted on the fact that the TRIPS Agreement sets a minimum standard of protection and Brazil is not obliged to protect above such threshold. In addition, Brazil would have a major disadvantage to guarantee access to drugs, since the extension of patent protection would benefit private interests, having negative repercussions for society, and therefore affecting public health services in the country and the citizen’s right to health.
On May 12th, 2021, the STF declared that the sole paragraph of article 40 of the Brazilian Intellectual Property Law was unconstitutional. The STF considered that all granted patents will be valid for twenty years counted from the filing date (and fifteen years for utility models), regardless of the time spent by the BRPTO to examine such applications.
In general terms, this decision will have ex nunc effects, meaning that those patents with the 10-year minimum term of protection granted by Article 40 which have already been granted before the publication of the final hearing minutes, will remain valid and in effect. There are, however, certain exceptions, as the effect will be ex tunc (retroactive) for the following:
• Patents and Certificates of addition comprising pharmaceutical products and processes, and equipment and/or materials for use in the medical industry;
• Patents with pending invalidity lawsuits grounded on the unconstitutionality of the sole paragraph of Article 40 and filed by 7 April 2021;
• Patents sent to ANVISA for prior approval, related to article Art. 229-C of the Brazilian IP Law;
• Patents having at least one of the following international classifications: A61B, A61C, A61D, A61F, A61G, A61H, A61J, A61L, A61M, A61N, or H05G, (considered by WIPO as related to medical-technologies);
• Patents having at least one of the following IPCs: A61K/6, C12Q/1, G01N/33, G16H;
• Patents with published lawsuit decisions (specifically published under Brazilian Code 19.1);
From May to June 2021, the BRPTO has already published three lists on the Official Bulletins:
- First list comprising patents with prior consent granted by ANVISA - 3,341 pharmaceutical patents were affected;
- Second list comprising patents having at least one of the following international classifications: A61B, A61C, A61D, A61F, A61G, A61H, A61J, A61L, A61M, A61N, or H05G, (considered by WIPO as related to medical-technologies) – 2,114 medical-technologies patents were affected;
- Third list comprising patents having at least one of the following IPCs: A61K/6, C12Q/1, G01N/33, G16H – 97 patents were affected;
Jointly with the referred third list, the BRPTO also published an official communication stating that holders can file arguments requesting the BRPTO to restore the original patent term, which must be filed within 60 (sixty) days counted from the publication of the corresponding correction.
It is important to note that if the BRPTO accepts the arguments, the term of protection will be restored to its original term considering the granted term by the Sole Paragraph of Article 40 of the Brazilian Intellectual Property Law.
The BRPTO will continue publishing additional lists of patents affected by the STF decision.
Final Remarks
If the BRPTO continues with the results achieved during the last 2 (two) years, which have enabled the reduction of the average time to grant a patent, the sole paragraph of article 40 would naturally fall into disuse.
During the 5 (five) years of discussion around ADI 5529 the STF received Amicus Curiae petitions supporting the constitutionally of Article 40’s sole paragraph, arguing (i) that the purpose thereof was to comply with article 62.2 of TRIPS and (ii) that protection changes could generate legal uncertainty to innovative companies. However, the Amicus Curiae petitions against the sole paragraph were more relevant, supported by arguments indicating that its provisions could interfere in a free competition market and therefore damage the national industry, public health and individuals by preventing access to drugs.
Although as discussed, the STF decision, which justified its decision on the fact that the protection of private rights and public interests must be balanced, will produce effects on a large number of patents and patent applications, its major effects will be observed on the pharmaceutical sector which will experience ex tunc effects. It is expected that these companies will legally challenge the decision in order to preserve their rights along with requesting fast-track procedures not only in the Intellectual Property sphere, but also in the regulatory sector.
Also relevant, as aforementioned, the BRPTO has informed that after the publication of the term of protection correction, the patent holders will have the opportunity to file arguments requesting for the BRPTO to revise its act, and if the arguments are considered as relevant, the term will be readjusted to the original term.
UNGRIA is an Intellectual Property law firm, founded in 1891, with 15 offices around 5 different jurisdictions. Our offices in Spain, Brazil, Mexico and Argentina provide direct filing, prosecution and litigation capabilities in Europe and Latin America for both patent and trademark work, coupled with local supervision and coordination oversight in New Jersey, as a “one point of contact”, to numerous IP teams of Fortune 500 companies and some of the most reputed IP law firms in the U.S.
If you have any questions or would like more information, please contact Jose Vazquez at jose.vazquez@ungriausa.com.
References
-Constituição da República Federativa do Brasil de 1988. planalto.gov.br. (n.d.). http://www.planalto.gov.br/ccivil_03/constituicao/constituicaocompilado.htm.
-Instituto Nacional da Propriedade Industrial. (n.d.). Estatísticas Instituto Nacional da Propriedade Industrial. Instituto Nacional da Propriedade Industrial. https://www.gov.br/inpi/pt-br/central-de-conteudo/estatisticas.
-Paranhos, J., Mercadante, E., & Hasenclever, L. (2020, November). O custo da extensão da vigência de patentes de medicamentos para o Sistema Único de Saúde. ResearchGate. https://www.researchgate.net/publication/346924792_O_custo_da_extensao_da_vigencia_de_patentes_de_medicamentos_para_o_Sistema_Unico_de_Saude.
-Supremo Tribunal Federal. (n.d.). Supremo Tribunal Federal. https://portal.stf.jus.br/processos/detalhe.asp?incidente=4984195.










